Welcome to Genetix
Pharmaceuticals
Genetix’ Mission is to develop and commercialize effective gene therapies for the treatment of serious, chronic human diseases and cancers.
Since inception, GENETIX Pharmaceuticals has positioned itself as a leader in the stable transfer and expression of therapeutic genes into a broad range of target cells resulting in effective, long-term gene therapy.
GENETIX’ gene transfer technology represents an unparalleled platform for the effective therapeutic human delivery of the Company’s proprietary genes for genetic blood disorders as well as genes for the correction of severe chronic disorders such as macular degeneration and neural degenerative disorders.
Lentiviral Gene Therapy
Genetix’ key proprietary technology is a recently developed, next generation gene therapy vector. This modified lentivirus, extensively engineered to be safe for human use, capitalizes on the unique and powerful attributes of the lentivirus. It has achieved unprecedented levels of stable transduction of target cells in multiple in-vitro and in-vivo preclinical model systems, including non-dividing primary human cells.
Genetix will utilize this vector system to develop both in-vivo and ex-vivo gene therapies for a variety of chronic diseases afflicting man, including inherited blood disorders as well as degenerative diseases of the eye and brain.
Functional Genomics
To support its mission in gene therapies and to provide a productive source of proprietary therapeutic genes, Genetix is focused on developing functional genomics research. This approach is expected to generate large numbers of new, therapeutically useful genes within the next several years. Genetix has acquired a broad option for multiple exclusive licenses on all discoveries in the areas of leukemias and lymphomas.
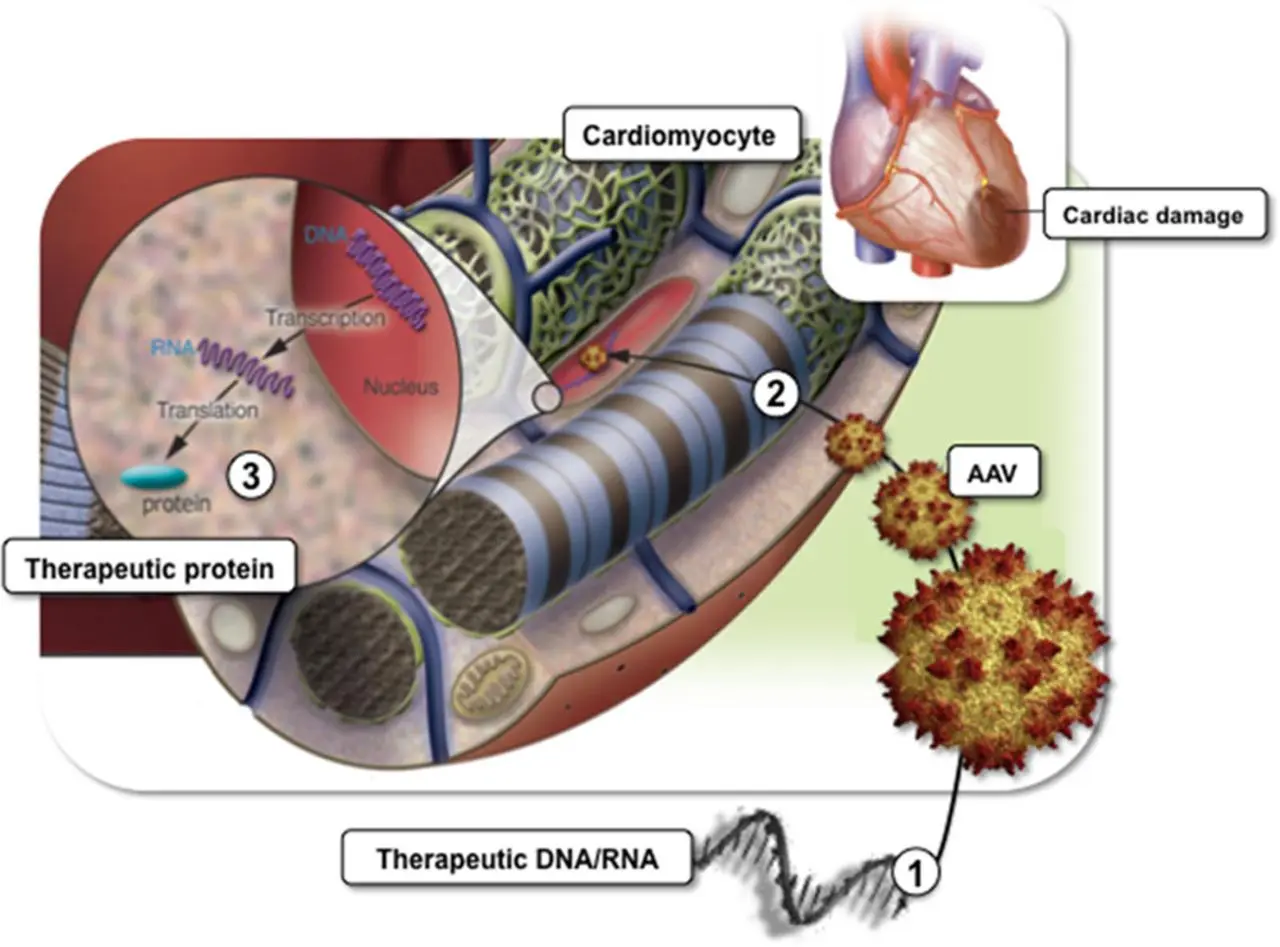
Cardiovascular Therapy
Genetix is focused on the identification of angiogenesis-promoting gene vectors and biologics for the treatment of coronary artery disease. A novel approach has been identified and is currently in advanced pre-clinical testing.